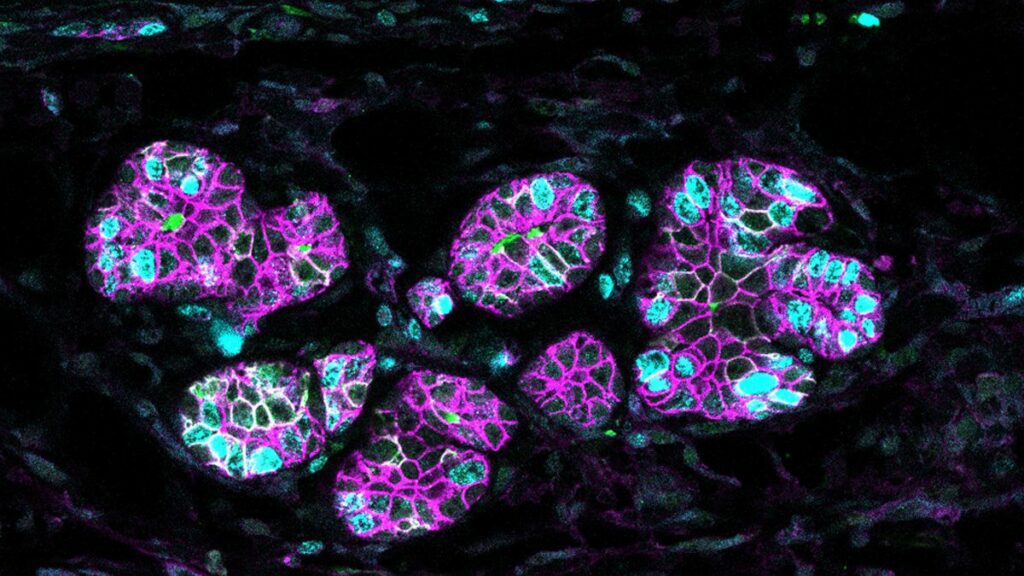
Milk production in mammals is a crucial process for the organism, perfected along the course of evolution. It is regulated by sophisticated molecular mechanisms still under investigation. A new study published in Nature Communications comes a step further towards understanding them, as it delves into the role of a protein, called RANK, which is essential for the functionality of mammary glands, and is also key in breast cancer.
This milk production takes place in the mammary epithelial tissue, which consists of two main cell types: luminal and basal. Luminal cells line the first layer of the mammary ducts and are involved in the actual milk production; the basal cells surround the luminal cells and cause the contraction that make milk flow during lactation.
Notably, both luminal and basal cell lineages originate from basal cells, which differentiate into luminal ones in the embryo. However, they become specialized after birth: basal cells only generate basal ones, and luminal cells only generate luminal ones.
A protein involved in breast cancer
The new study, led by Eva González-Suárez, head of the Transformation and Metastase Group at the IDIBELL and the CNIO, describes the behaviour and interaction of these two cell lineages during pregnancy and lactation, as well as the role therein of RANK protein.
This protein has been the focus of González-Suárez’s interest sine she discovered in 2010 that it plays a key role in breast cancer development. Her group currently receives funding from the European Research Council (ERC) to develop a new generation of drugs against breast cancer aimed at blocking RANK-mediated signaling.
The main contribution of the study now published, highlights González-Suárez, is “the creation of animal models with the RANK protein suppressed only in either luminal or basal cells. Thus, we have been able to discriminate its function in each of the cell types”, something unknown so far.
The study shows that the consequences of RANK suppression on mammary gland functionality are different depending on whether the mice experience their first or second pregnancy, or whether they have not yet been pregnant.
No milk on the first pregnancy, but on the second
When RANK is eliminated from luminal cells before any pregnancy has ocurred, these cells start an aberrant differentiation into another type of cells responsible of milk formation, called alveolar cells. The first lactation fails, though, as these aberrant alveolar cells are unable to produce milk.
In the second pregnancy, luminal cells turn out to be even more dysfunctional. This alerts basal cells, which gain back their capacity to differentiate into luminal alveolar cells, now competent to produce milk. Lactation, in this second pregnancy, becomes then successful. In other words, thanks to this “rescue action” of basal cells, which do in fact express RANK, a functional lactation takes place. “This bipotency capacity of basal cells can arise in a physiological context, in order to restore such an important process as lactation”, González-Suárez explains.
For the author responsible for the study, “it is very interesting that, in the absence of RANK, luminal cells differentiate into alveolar ones in an aberrant manner, even in female mice that have not yet entered pregnancy. And this difference between what happens during the first and the second pregnancy is also relevant”. Regarding the possibility that these processes may have a parallelism in human lactation, she considers it “probable, although further studies would be necessary to determine it”.
The main co-authors of the paper are the postdoctoral researcher Ana Sofía Rocha, from Biomedical Research Institute Bellvitge (IDIBELL), also co-leader of the study, and Alejandro Collado-Solé, a doctoral student at the Spanish National Cancer Research Centre (CNIO), both from Eva González-Suárez’s group.